
Privacy statement: Your privacy is very important to Us. Our company promises not to disclose your personal information to any external company with out your explicit permission.
1.Introduction
Ceramic to ceramic or ceramic to metal joining have been using high vacuum technology, medical technology, electrical and sensor industries. Brazing is often the preferred method for joining process because of its capability for joining different types of materials, high reliable and precision production tolerance. To join ceramics to itself or to metal, the critical problem is the poor wettability of ceramics by filler alloys. This problem is usually overcome by applying a metallic layer on the ceramic surface. The most widely known
metallization process is the “Moly –Manganese” process.
The article is to investigate the variation of the adhesion strength of the Al 2 O 3 /Mo-Mn interfaces as the changing of joining temperatures. The adhesion between Mo-Mn layer and Al2O3 is determined by four-point
bending test. The adherence mechanism
and adhesion strength at different
joining conditions is then determined
and characterized.
2. Experiments
An aluminium oxide ceramic powder was used in this experiment. It was uniaxially pressed in a steel mold at 13 MPa to make rectangular specimen for the subsequent joining process. After firing at 1625℃ the relative density of alumina specimen approached to a value of 99% alumina.The preparation of the Mo-Mn paste involves of homogenously mixing of powders and polymers (72-28 % wt).
Final dispersion and mixing of Mo-Mn paste was carried out with ball milling and three roll mills.
The screen printing process was adopted
to fabricate the metallization layer. The joining process was performed at temperatures ranging from 1100℃ to 1470℃ in 95%N 2 -5%H 2 . The
determination of adhesion strength was based on the four-point bending test.The proposed sample geometry is a modification of the four-point bending geometry suggested by Charalambides [2], as shown schematically in Fig. 1.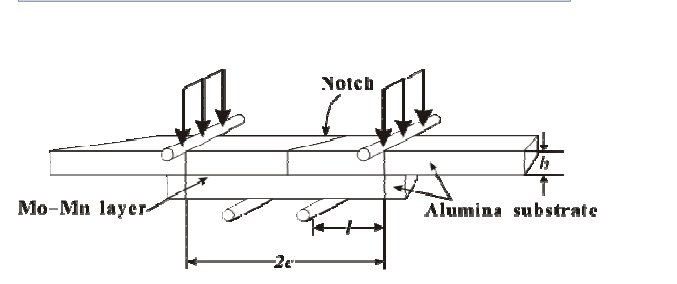
Fig. 1. Modified notched four-point bending specimens
3. Results and Discussions
3.1 Microstructure
The microstructures of cross section resulting from various joining temperature are shown in Fig. 2-5. The joining temperature affects bonding
results in two major aspects: higher temperature encourages the densification of Mo and the diffusion of MnO and glassy phase from the Mo-Mn layer into alumina matrix.
Fig. 2 shows the cross section of the specimen obtained at 1100℃, the white and dark phase constituents are Mo and Al 2 O 3 , respectively. The grey phase is the mixture of Mn-,
Ti-containing glassy phase as detected
by EDS. For the specimen A3, the Mo-Mn layer reveals a homogeneous structure with some glassy phase. Besides, the glass fills into the pores of the Mo-Mn layer.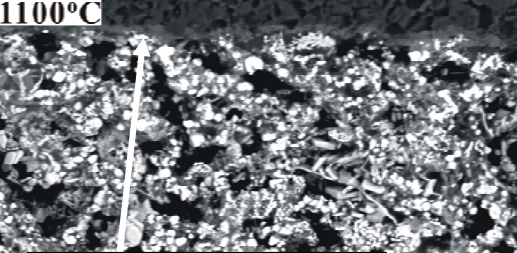
Figure 2: Interfacial microstructure of
jointing area (1100 ℃ )
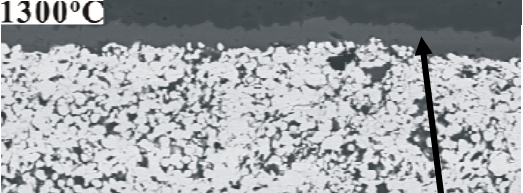
Figure 3: Interfacial microstructure of joining section(1300 ℃ )
As can be seen from Fig. 2-3, the glass wets the ceramic and flows along the interface leading to a uniform reaction layer. EDS analyses
demonstrates that the composition of this layer is closed to MnAl2O4 . The formation of a MnAl 2 O 4 layer is association with MnO formation. The
adherence mechanism of the Mo-Mn/Al 2 O 3 interfaces is adquately studied by Pincus [4-5]. He suggested that manganese is oxidized to MnO in a reduction atmosphere and then reacts
with alumina, forming a MnAl 2 O 4 layer
along the interface. However, the high joining
temperature of 1470℃ gives rise to the
diffusion of MnO-containing liquid phase into alumina matrix. For this reason, the MnAl 2 O 4 layer dissappears for the specimen B5. This also means the Mo-Mn layer is depleted of glassy
phase. As discussed in Ref. [3-4], this effect would be expected to promote the sintering of Mo, and eventually lead to the densification and grain growth of Mo, as shown in Fig. 4 (a).
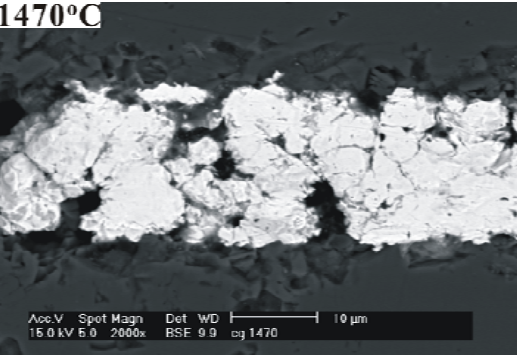
3.2 Analysis of load-displacement
curves
A typical load-displacement curve
is shown in Fig. 5.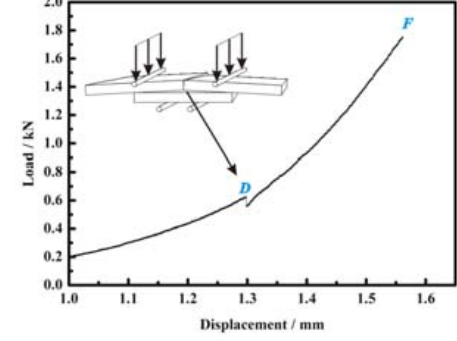
As expected, the load initially increases linearly. In contrast to pervious studies on the four-point
bending test by other authors [2], the load-displacement curve did not reveal the load plateau, which corresponded to the steady-state crack extension along the interface. On the contrary, the load-displacement curve shows two
characteristic points, designated as D and F. The separation of Al 2 O 3 /Mo-Mn interface started at point D.That is, the stress ahead of the interface near the central part of the specimen is large enough to generate an interfacial debonding at the interface.Therefore, we can treat point D as the critical load for interfacial debonding force on the specimen increases
continuously with bending moment.Eventually, the specimen interfaces fail at point F.
near the central region of the specimen.Although the critical load D recorded in the load-displacement curve can not be used to calculate the strain energy release rate, it reflected the fracture resistance of the interface of interest.
The adhesion strength results are shown
in Fig. 6.
After the applied load reaches point D, it dropped sharply as crack advances since the compliance increases. The force on the specimen increases continuously with bending moment. Eventually, the specimen interfaces fail
at point F.
3.3 Adhesion strength
The adhesion strength results are shown in Figure. It shows large data scatters probably reflecting the inhomogeneities of Al 2 O 3 /Mo-Mn interfaces. However, just considering the
mean values, the trends of adhesion strength change with temperature is shown in Fig. 6. The results do not exhibit monotonic trend in the joining temperatures range. That is, the
temperature had strong impacts on the
joint properties. The variation of joining
temperature influenced the joint strength
in following aspects:
(i) Fig. 2 indicates that the majority of Mo particles bonded to the glassy phases and only some particles form contacts with each other and results in inconsistent structure. However, raising the joining temperature to 1300℃
resulted in the dense Mo-Mn layer (Fig.3). Besides, the glassy phases fill the pores and form strong bonds to both Mo-Mn layer and Al 2 O 3 [3,6]. It shows no evidence that the diffusion of MnO and glassy phase from the Mo-Mn layer into the Al 2 O 3 substrate and results in well-bonded interfaces between Al 2 O 3
and Mo-Mn layer. (ii) Fig. 4 (a) shows that some
cracks and defects are found inside the Mo-Mn layer. The presence of these cracks and defects indicates the high brittleness of the Mo grain boundaries. It is supposed that; when the joining
process is carried out at 1470℃, the residual glassy phase in the Mo-Mn layer softened and flow along the grain boundary of Mo. Consequently, it precipitates at the grain boundaries. However, for the specimens whose
joining temperatures are well below 1470℃, the glassy phase fills into the pores of the Mo-Mn layer rather than as the precipitations. Because of that the grain boundary acts as an effective
obstacle to crack extension when subject to stress, the precipitation of glassy phases along the Mo grain boundaries would be expected lower its strength.
(iii) For the specimens joined at 1100℃ and 1300℃, a uniform MnAl2O4 layer forms and thus develops well-bonded interfaces. Fig. 4 indicates that the MnO and SiO 2 diffused past the interface into the alumina matrix;
however, which leads to the vanishment of MnAl 2 O 4 layer. The absence of MnAl 2 O 4 layer is because that, the joining temperature is too high such that the MnO-containing glassy phase
penetrated into the alumina matrix rather than reacted with the Al 2 O 3 at the interfaces. It results in a weak interface between Al 2 O 3 and Mo-Mn layer. Influences by the above factors, the overall effect of temperature on the
adhesion strength can be concluded as
follow:
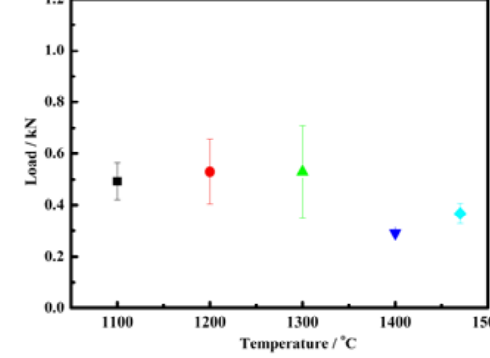
A slight increase in adhesion strength is observed as the joining temperature rising from 1100℃ to 1300℃. This is because that the dense Mo-Mn layer can provide more resistance to crack extension. In the meantime; for the specimen joined at 1300℃, the glassy phases can fill into pores in the Mo-Mn layer and provide mechanical locking between Al 2 O 3 and
Mo-Mn layer. However, the adhesion strength rapidly drops when joining process is preformed at higher temperature (1470℃). This is attributed to the poor adherence between the Al 2O3 and Mo-Mn layer, which results from
the absence of MnAl 2 O 4 layer. Besides, brittleness of glassy phase at Mo grain
boundaries also significantly reduces the
fracture resistance.
4. Conclusions
The joining temperature affects microstructure in two major aspects: higher temperature encourages the densification of Mo and the diffusion of MnO and glassy phase from the Mo-Mn layer into alumina matrix. The adhesion
strength results strongly depended on the
joining temperatures. The adherence mechanism is associations with a MnAl2O4 layer formation. The absence of MnAl 2 O 4 layer results in a weak interface between Al 2 O 3 and Mo-Mn
layer.
January 19, 2022
January 03, 2017
December 09, 2023
August 12, 2022
November 06, 2024
November 07, 2024
January 15, 2024
January 15, 2024
As the microstructure of the surface of advanced ceramic materials is different from the metal materials, brazing often does not happen from ceramic to metal to bond them directly. Therefore, the...
1. In the raw materials, it breaks through the boundary of traditional ceramics with clay as the main raw material. Special ceramics generally use oxides, nitrides, silicides, borides and carbides as...
Oxygen evolution reaction (OER) is an important half-reaction in energy conversion and storage devices such as electrolysis of water and secondary metal-air batteries. However, OER has a high...
With the continuous development of science and technology, the progress of industrial ceramics has also made many breakthroughs,especially the research and development of multiphase composite...
Email to this supplier
January 19, 2022
January 03, 2017
December 09, 2023
August 12, 2022
November 06, 2024
November 07, 2024
January 15, 2024
January 15, 2024

Privacy statement: Your privacy is very important to Us. Our company promises not to disclose your personal information to any external company with out your explicit permission.

Fill in more information so that we can get in touch with you faster
Privacy statement: Your privacy is very important to Us. Our company promises not to disclose your personal information to any external company with out your explicit permission.